A Gateway to Therapeutic Discovery and Immune Tolerance
HLA-G
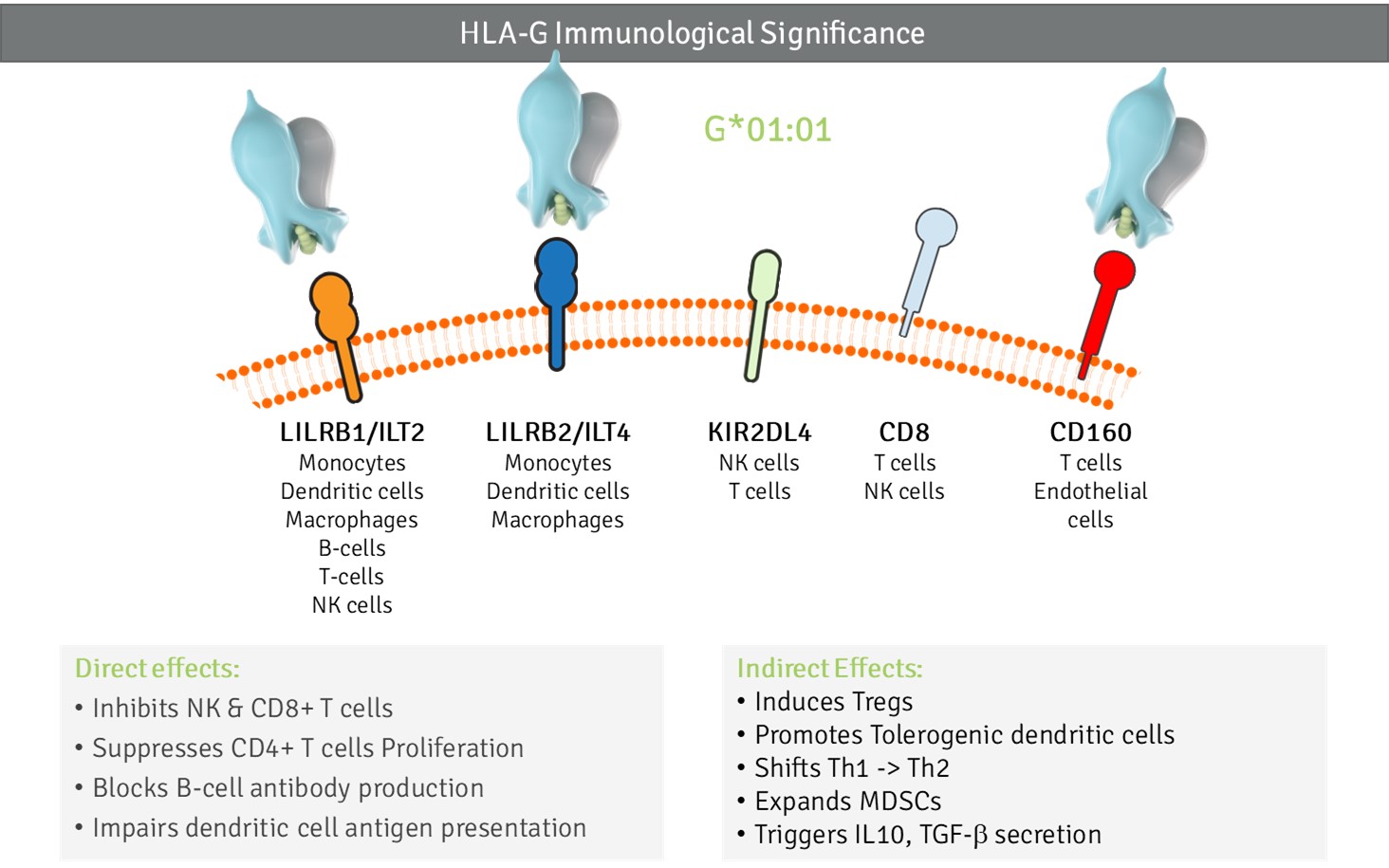
Immunological Significance of HLA-G
1. Restricted Polymorphism and Distinct Function
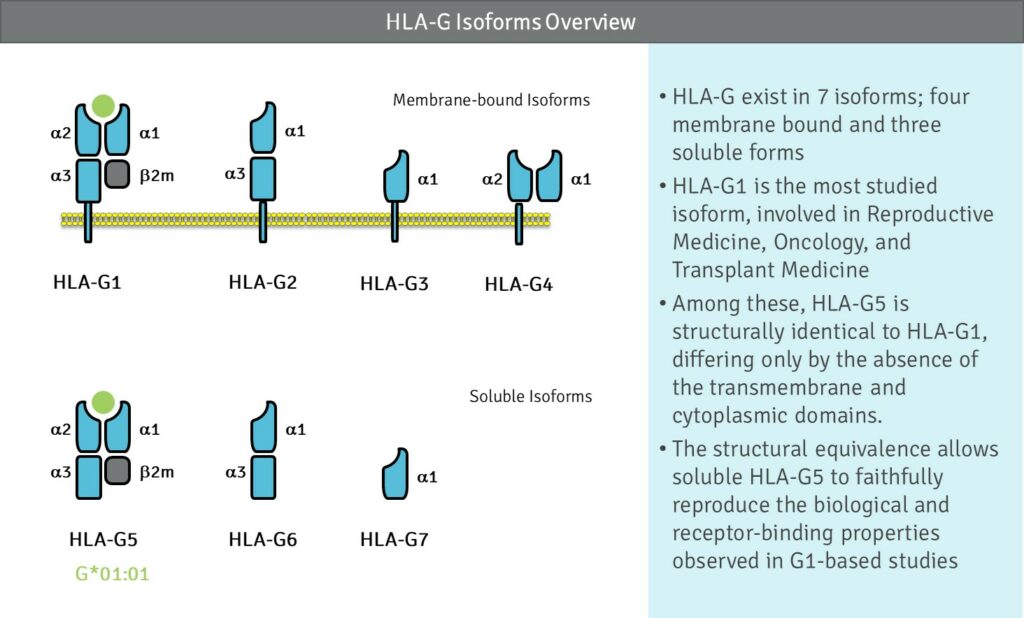
HLA-G stands apart from classical MHC molecules through its limited polymorphism—fewer than a hundred alleles compared with thousands for HLA-A, -B, and -C. This low variability narrows its peptide-binding profile, favoring self-derived intracellular peptides that transmit a tolerogenic signal rather than a classical immune activation cue. Under normal conditions, expression is restricted to immune-privileged tissues such as the placenta, thymus, cornea, pancreatic islets, and activated monocytes—defining its role as a guardian of immune quiescence.
HLA-G’s minimal diversity and controlled expression mark it as a molecular cornerstone of immune tolerance.
2. Immune Checkpoint Control
Functionally, HLA-G acts as a broad-spectrum immune checkpoint. Through high-affinity interactions with ILT2, ILT4, KIR2DL4, CD8, and CD160, it suppresses effector functions of NK cells, T cells, and B cells, while inducing tolerogenic dendritic cells and regulatory T cells. Soluble HLA-G isoforms extend these effects systemically, providing durable immune suppression in both physiological states (pregnancy, tissue repair) and pathological settings (cancer, infection, transplantation).
HLA-G integrates local and systemic immune control, shaping tolerance through both cell-bound and soluble pathways.
3. Translational Relevance and Research Value
By balancing immune activation and inhibition, HLA-G represents a biological model of immune regulation. Its mechanisms underpin translational research in:
- Tumor immune evasion and checkpoint blockade strategies
- Graft tolerance and transplant immunology
- Infectious and inflammatory disease modulation
- Autoimmunity and reproductive immunology
Understanding HLA-G provides actionable insight for developing diagnostics, biologics, and immunotherapies that harness natural tolerance.
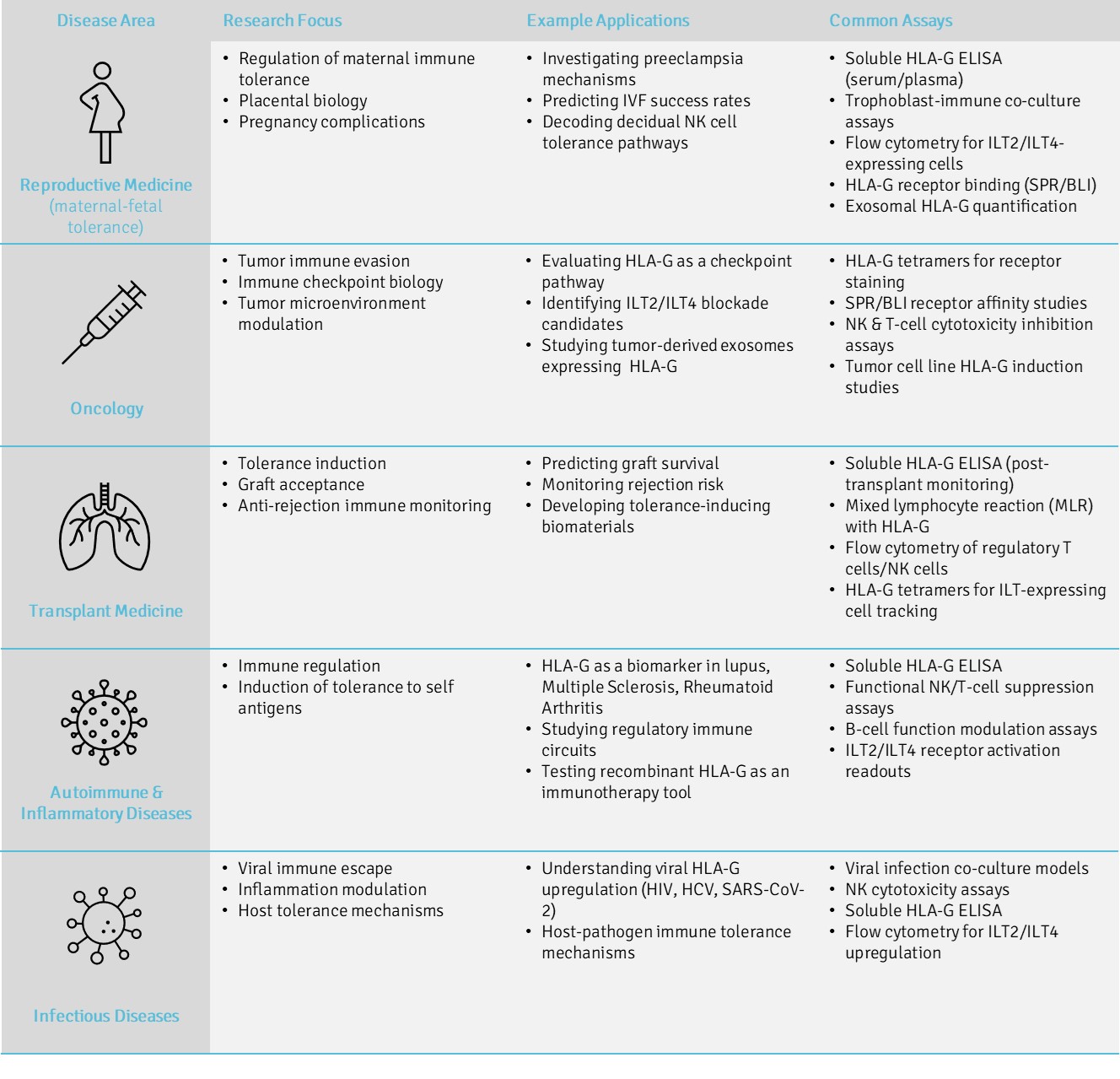
Disease Fields and Research Focus
HLA-G serves as a unifying model for studying immune regulation across a wide spectrum of biological and clinical systems. Its tolerogenic mechanisms—ranging from modulation of NK and T-cell responses to interaction with inhibitory receptors such as ILT2 and ILT4—make it a pivotal molecule in both physiological and pathological contexts.
Across research fields, HLA-G provides a functional bridge between fundamental immunology and therapeutic innovation. In reproductive medicine, it defines the immune balance critical for successful implantation and pregnancy. In oncology, it marks a major axis of tumor immune evasion and checkpoint control. In transplantation, it contributes to graft acceptance and donor-specific tolerance.
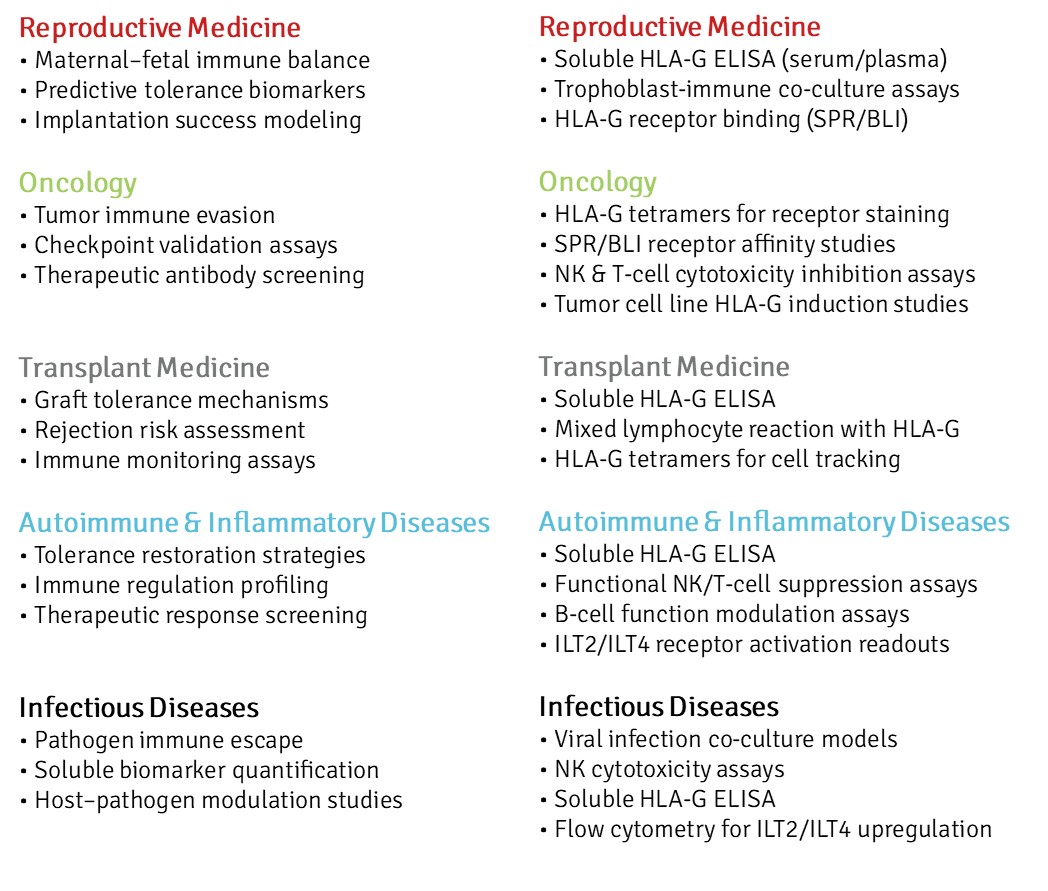
Our recombinant HLA-G reagents, validated for structure, receptor binding, and biological activity, enable standardized investigations in these and related domains. The following areas illustrate how our platform supports precision immunology and translational research—from mechanistic assays to biomarker development and therapeutic screening.
Reliable HLA-G reagents advance research, biomarker discovery, and therapy development across multiple fields.
HLA Protein Technologies Differentiation
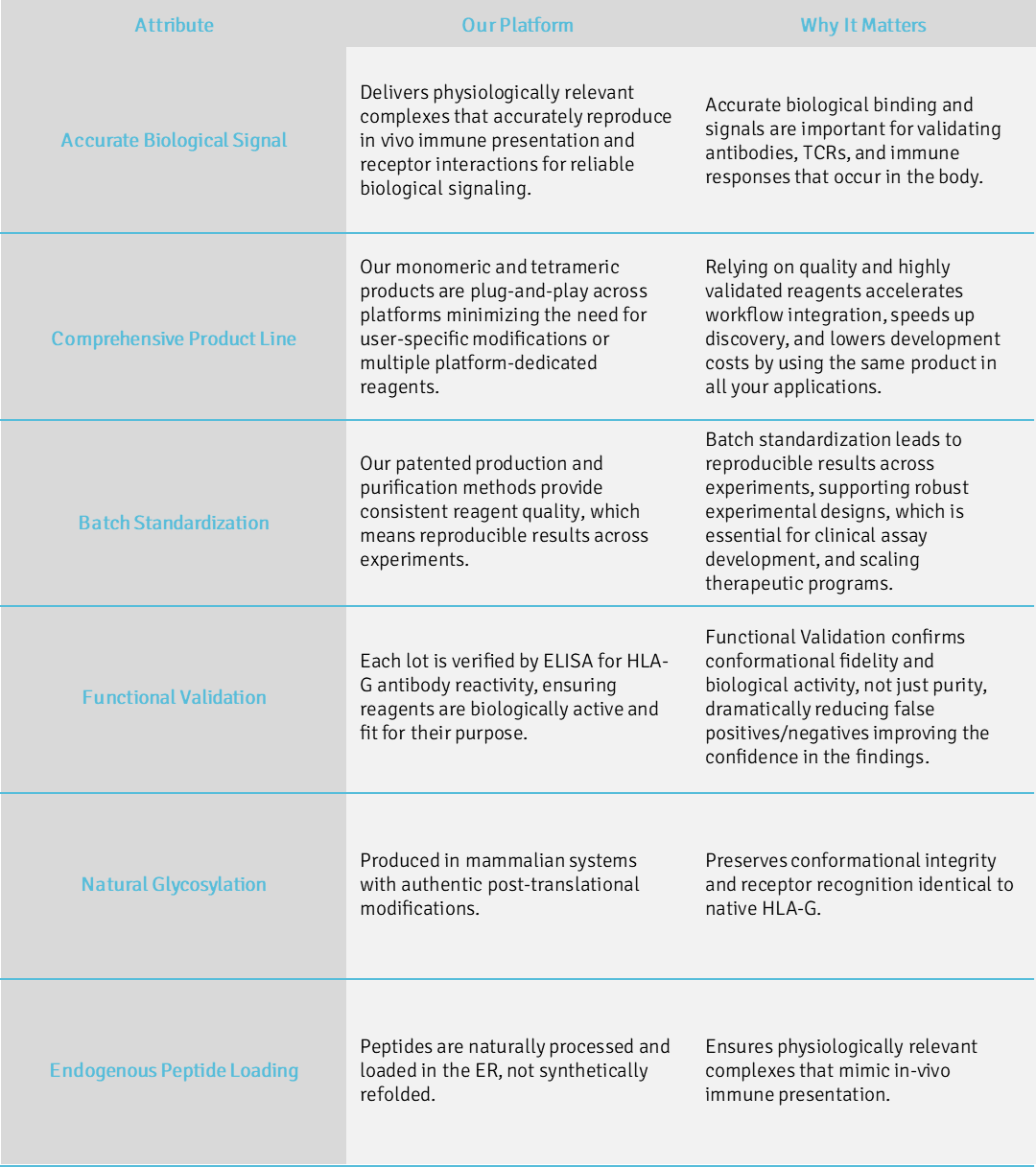
Our proprietary mammalian expression platform delivers soluble, naturally folded, and authentically glycosylated HLA-G molecules that preserve physiological peptide loading. This native-like architecture ensures unmatched reliability, overcoming the limitations of traditional recombinant HLA production. We provide reagents that faithfully reproduce the true biological context—driving confidence and consistency in every experimental result.
These biochemical and structural features translate directly into experimental reliability. Researchers gain reagents that mirror the true native HLA-G environment—critical for any study of immune checkpoint modulation, receptor binding, or antibody development.
Translating native biology into dependable research performance.
Cross-Disciplinary Research Integration
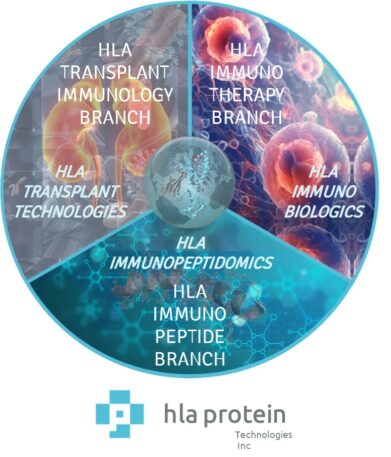
Our HLA-G technology platform bridges immunology, structural biology, and translational discovery through three complementary application branches. Each branch harnesses authentic, endogenously loaded HLA-G molecules to deliver biologically faithful insights across antibody validation, peptide binding, and receptor engineering. Together, these approaches enable reliable translation from molecular mechanism to therapeutic innovation—advancing research in transplantation, immunopeptidomics, and immunotherapy with unmatched precision and reproducibility.
A single platform powering discovery across every branch of immune research.

Functional Validation and Quality Control
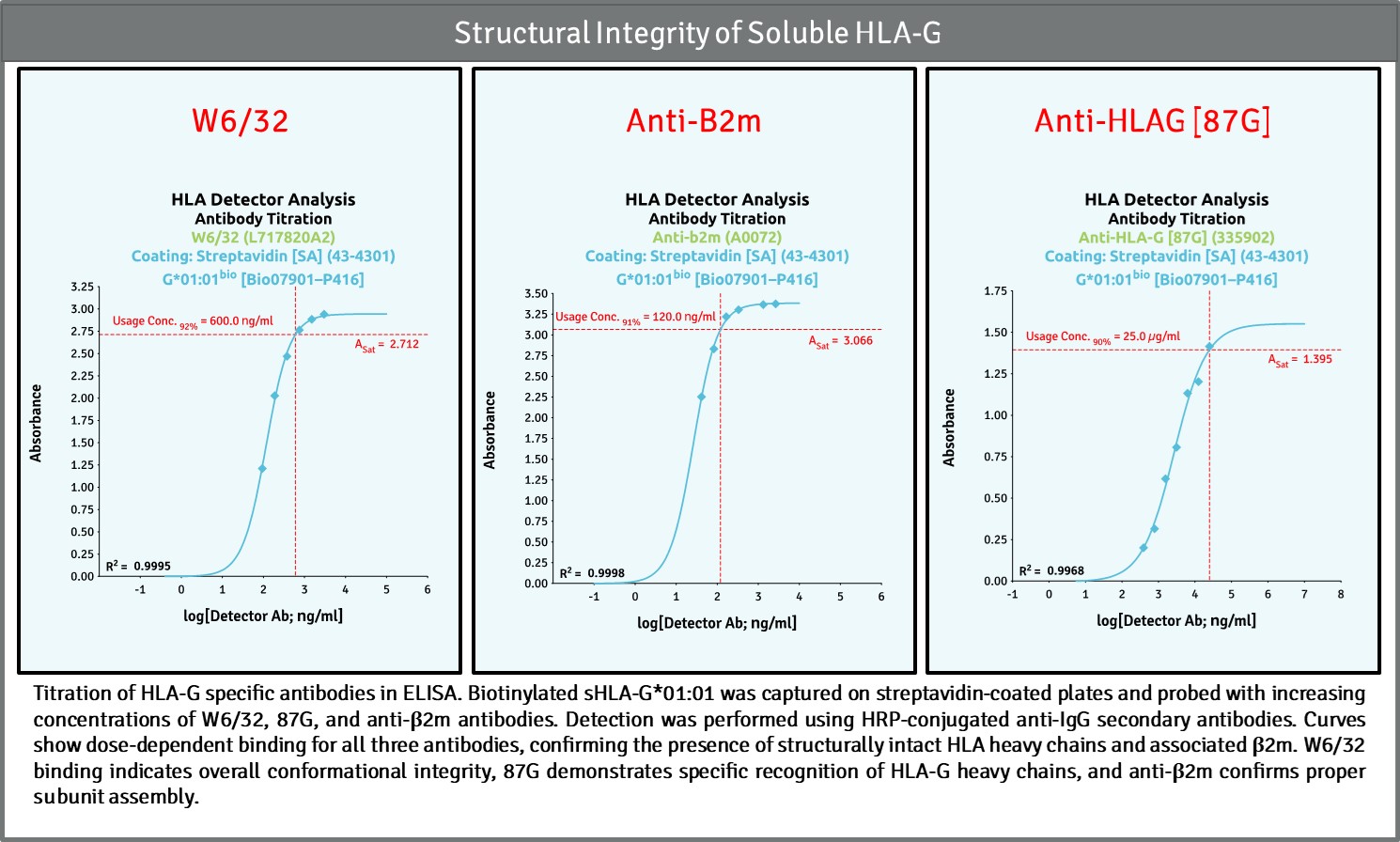
Our validation platform ensures every HLA-G molecule—whether non-modified or biotinylated—is structurally intact, biologically active, and consistent across batches. Using a proprietary sandwich ELISA (swELISA) built around the conformational antibody W6/32 and β₂-microglobulin recognition, we confirm correct folding, stability, and native complex formation of each HLA molecule.
Through dose-response potency analysis (EC₅₀ modeling), we quantify functional integrity and detect even subtle deviations in structure or activity. This approach provides traceable, reproducible data that define Critical Quality Attributes (CQAs) such as antigenicity, purity, and stability—core metrics for translational and diagnostic reliability.
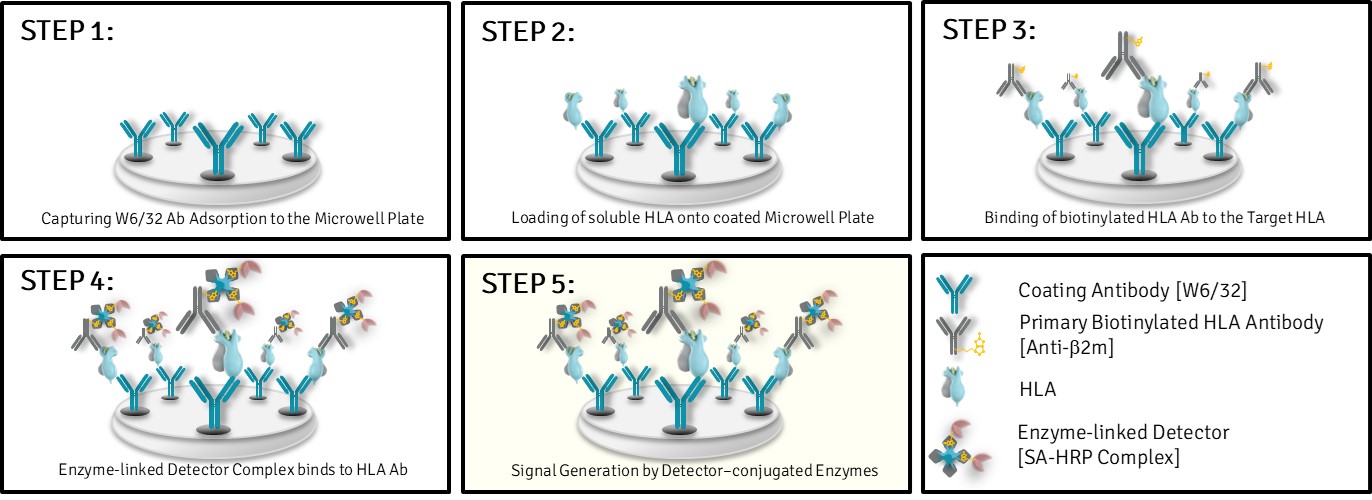
ELISA Workflow at a glance for Non-modified HLA-G*01:01:
- Capture of non-modified sHLA-G via W6/32 (conformational epitope)
- Detection using biotinylated anti-β₂m and streptavidin-enzyme conjugate
- EC₅₀-based potency quantification and structural integrity confirmation
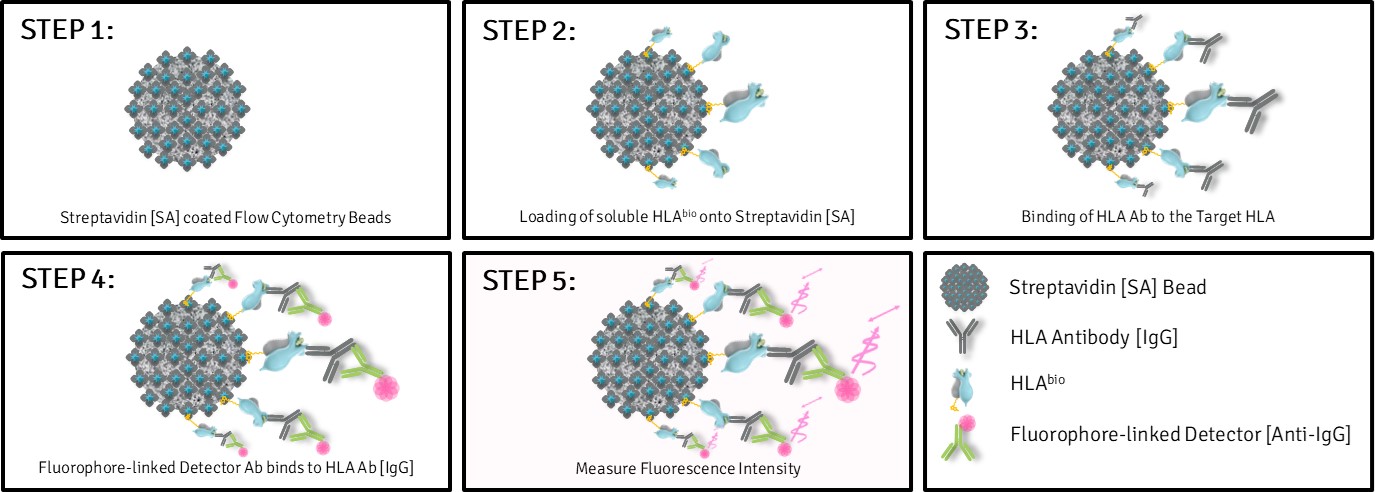
Luminex Workflow at a glance for Biotinylated HLA-G*01:01:
- Capture of biotinylated sHLA-G via streptavidin beads
- Detection using W6/32 and Fluorophore-linked detector
- EC₅₀-based potency quantification and structural integrity confirmation
Each assay is benchmarked against an internal reference standard, ensuring every lot meets analytical performance criteria for precision, sensitivity, and biological relevance.
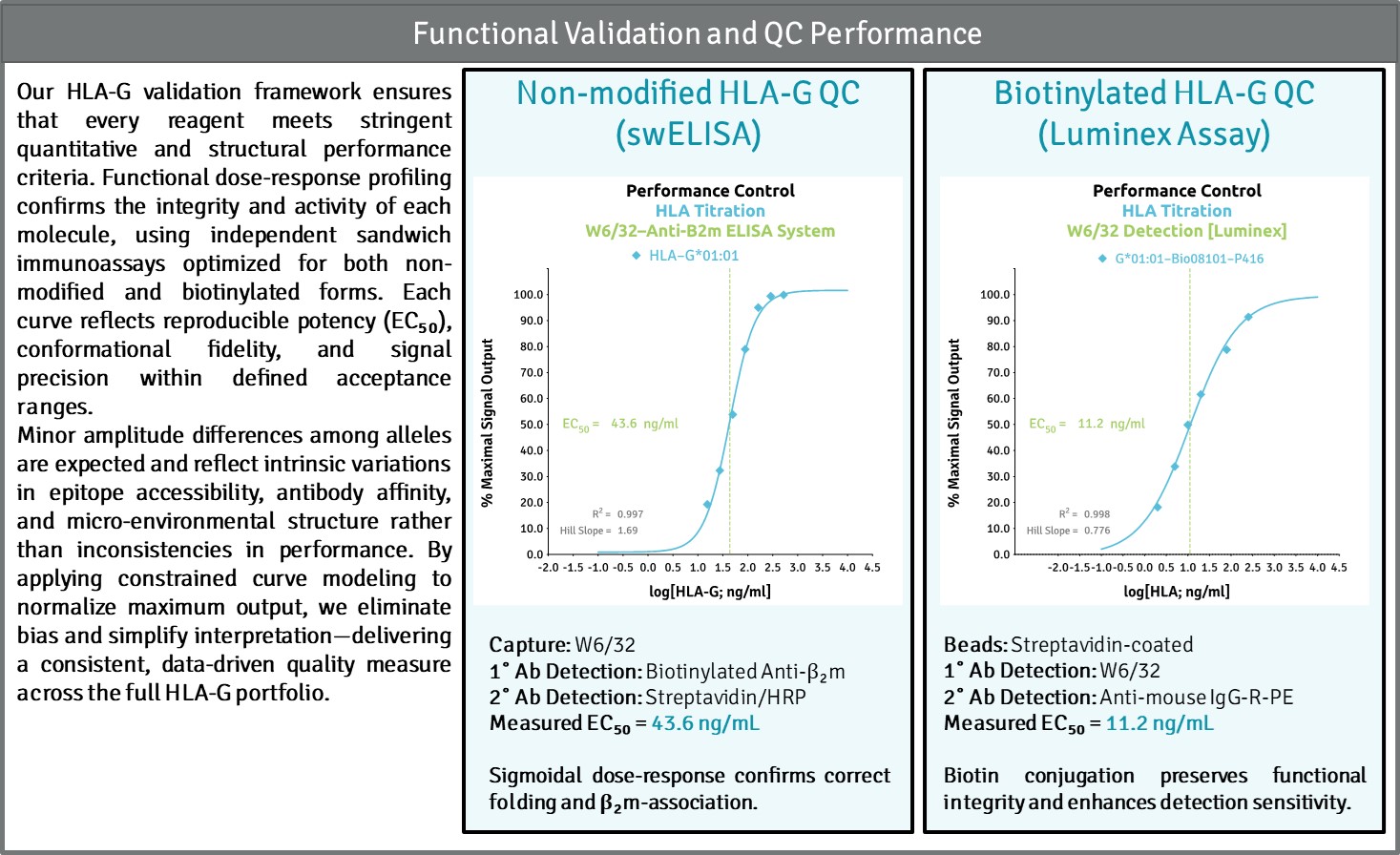
Precision-validated HLA-G—where structural integrity meets measurable performance.
How can we help?
HLA Protein Technologies Inc has developed a proprietary, novel approach for manufacturing of soluble Class I and Class II HLA proteins for research, diagnostic, and therapeutic applications.
We can help you to …
Define your own custom manufacturing needs
Do you need to:
- produce a sHLA in large quantities?
- acquire a sHLA molecule that is not listed?
- custom manufacture HLA proteins?
- utilize GMP manufactured sHLA?
Develop your own assay strategy
Do you need to:
- find suitable antibodies that recognize sHLA molecules?
- couple sHLA to a solid support?
- optimize your assay?
- decide on a sHLA standard?
Create your own diagnostics or therapeutics
Do you need to:
- develop a strategy that utilizes large panels of sHLA?
- acquire a commercial license?
- make your own assay platform?
- collaborate on a project?
Talk to an expert today
We understand the challenges you face. If you are still wondering if our technology can take you to the next level and are looking for some clarity, reach out to our experts and someone from our team will get back to you in no time.
Expert advice, whenever you need it!
Featured Resources
PUBLICATION
Profiling antibodies to class II HLA in transplant patient sera.
McMurtrey C, Lowe D, Buchli R, et al.
Hum Immunol. 2014 Mar;75(3):261-70.
PUBLICATION
Large-scale production of class I bound peptides: assigning a signature to HLA-B*1501.
Prilliman K, Lindsey M, Zuo Y, Jackson KW, Zhang Y, Hildebrand W.
Immunogenetics. 1997;45(6):379-85. [PMID: 9089095]